Associate Director, Commercialization Funding
Tel: (848) 932-4487
pragati.sharma@rutgers.edu

The HealthAdvance Fund®
Advancing Biomedical Innovations
Advancing Biomedical Innovations
As part of the Rutgers Optimizes Innovation initiative, HealthAdvance Fund® provides commercialization funding to assist the development of early-stage life sciences technologies and make them more attractive for continued follow-on investments from industry partners and external investors.
About the Rutgers HealthAdvance Fund®
HealthAdvance Fund® is the funding platform of Rutgers Optimizes Innovation (ROI) program established with a $4 million grant received under the National Institutes of Health (NIH) Research Evaluation And Commercialization Hub (REACH). The program aims to energize the innovation culture across all university campuses to speed up the translation of biomedical discoveries into commercially viable diagnostics, devices, therapeutics, and tools to improve health and patient care and train the next generation of innovators.
Pre-Qualification Guidelines & Form
Find out more about eligibility and application requirements for pre-qualification and the application process and timeline.

Meet the Mentors-in-Residence
Our Mentors-in-Residence (MIRs) are individually matched to the Innovator teams that have been invited to submit a full application to the program and collaborate with their matched Innovators to craft compelling proposals for a commercially viable product or solution, with a strong focus on improving human health.

Contact the Team
Executive Director, New Ventures
Tel: (848) 932-4551
vincent.smeraglia@rutgers.edu
Recently Funded Projects
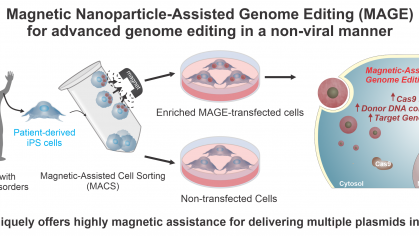
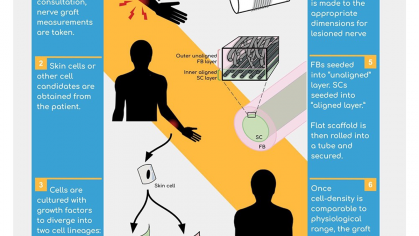
One of the most critical hurdles to developing CRISPR-based therapies for genetic disorders is the lack of effective delivery systems. To this end, this project aims todevelop a next-generation CRISPR delivery solution for fast and effective genome editing that supports the need for the CRISPR-based drug development market.
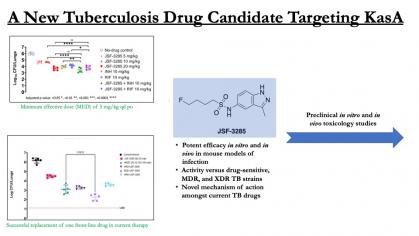
Tuberculosis is characterized by 10 million cases and 1.5 million deaths per year. New drugs are needed to reduce treatment duration and to treat drug-resistant infections. We propose a solution based on our preclinical drug lead (JSF-3285) that inhibits the essential ß-ketoacyl synthase and exhibits promising efficacy and safety profiles.
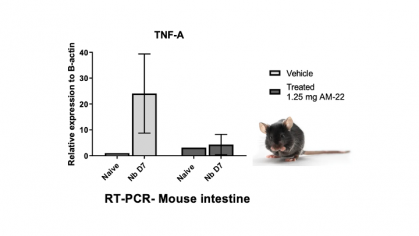
We have identified potent small molecule MIF inhibitors that reduce inflammation-associated cytokines in blood cell and mouse assays, and simultaneously exhibit favorable drug likeness properties including oral availability. They hold promise for the treatment of human inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease.
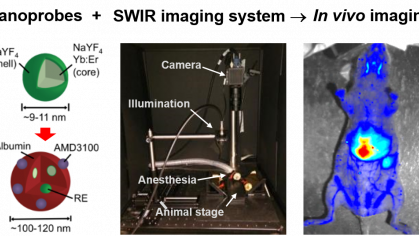
This project aims to advance nanoparticle-based contrast agents for pre-clinical imaging of tumors in small animals. The project focuses on (i) scaling up contrast agent synthesis, (ii) assembly of a prototype imaging system, and (iii) proof of concept studies to monitor tumor growth and response to therapy.
The prevention of microbial adhesion and biofilm formation on medical device/tissue interfaces is an urgent and unmet need. Thi surface functionalization technology, which is specifically effective in preventing biofilm formation on medical devices, such as implants, aims to address this critical healthcare problem.
The research team is developing a Cryptococcus fungal vaccine (HK-fbp1) that has shown cross protection against multiple major invasive mycoses, including Cryptococcus species, Aspergillus fumigatus, and Candida albicans. Its protection remains effective against Cryptococcus and Aspergillus in certain immunocompromised hosts, such as CD4 T cell-depleted mice, a condition mimicking AIDS patients.

Aberrant expression of bone morphogenetic proteins (BMP) has been shown to promote age-related diseases including cancer and Alzheimer’s disease. The research group has developed small molecular inhibitors targeting a BMP receptor that has never been targeted before, aiming to develop this novel class of compounds into a drug for the treatment of cancer and Alzheimer’s disease.