Associate Director, Commercialization Funding
Tel: (848) 932-4487
pragati.sharma@rutgers.edu

The HealthAdvance Fund®
Advancing Biomedical Innovations
Advancing Biomedical Innovations
As part of the Rutgers Optimizes Innovation initiative, HealthAdvance Fund® provides commercialization funding to assist the development of early-stage life sciences technologies and make them more attractive for continued follow-on investments from industry partners and external investors.
About the Rutgers HealthAdvance Fund®
HealthAdvance Fund® is the funding platform of Rutgers Optimizes Innovation (ROI) program established with a $4 million grant received under the National Institutes of Health (NIH) Research Evaluation And Commercialization Hub (REACH). The program aims to energize the innovation culture across all university campuses to speed up the translation of biomedical discoveries into commercially viable diagnostics, devices, therapeutics, and tools to improve health and patient care and train the next generation of innovators.
Pre-Qualification Guidelines & Form
Find out more about eligibility and application requirements for pre-qualification and the application process and timeline.

Meet the Mentors-in-Residence
Our Mentors-in-Residence (MIRs) are individually matched to the Innovator teams that have been invited to submit a full application to the program and collaborate with their matched Innovators to craft compelling proposals for a commercially viable product or solution, with a strong focus on improving human health.

Contact the Team
Executive Director, New Ventures
Tel: (848) 932-4551
vincent.smeraglia@rutgers.edu
Recently Funded Projects
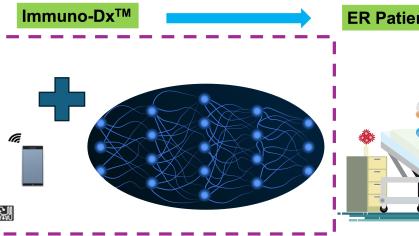
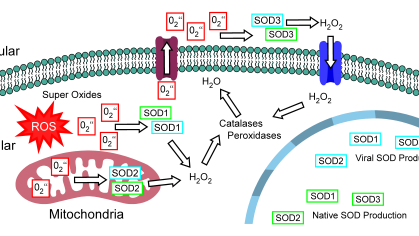
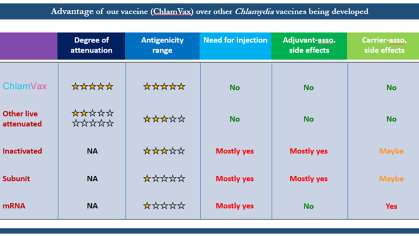
This project will develop a biomedical technology called Immuno-Dx, whose value proposition lies in its ability to provide vital information about patients’ immune response to infections, and to fulfill unmet need for personalized diagnostics of septic patients in emergency department (ED) settings.
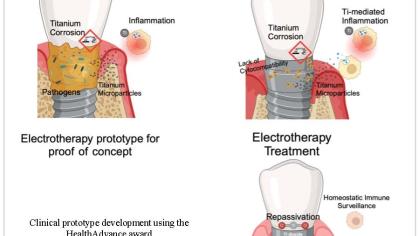
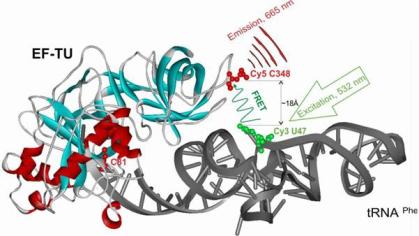
Our project aims to revolutionize peri-implantitis treatment by developing an innovative electrotherapy device designed to restore the passivation layer of titanium implants. By targeting the root cause of implant degradation and associated inflammation, we seek to enhance the longevity and success of dental implants and improve patient outcomes.
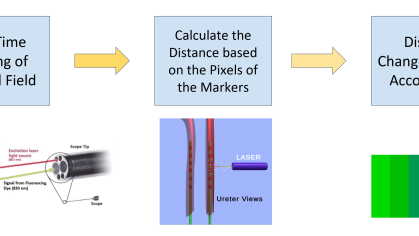
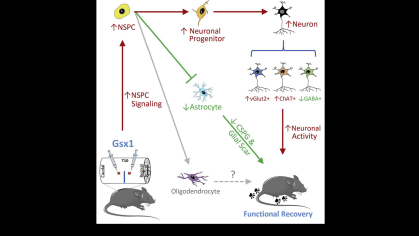
Rutgers researchers have developed an AI-based system to prevent iatrogenic ureteral injury in gynecologic and colorectal surgeries. The technology continuously tracks surgical instruments, providing real-time alerts or movement halts near the ureter. Platform-independent, it enhances intraoperative safety and surgeon control, reducing complications in complex cases with distorted anatomy.