Gsx1 Gene Therapy for Spinal Cord Injury
Inventor(s): Li Cai, Misaal Patel, Yi Lisa Lyu
Awarded: April 2023
Summary:
Spinal cord injury (SCI) is a devastating condition with permanent consequences that affects every aspect of a person’s life. Every year in the USA, there are ~18,000 new SCI cases. ~80% of SCIs occur in males aged 15-35 and more than half will experience some sort of long-term paralysis. Spinal cord injury patients often suffer from life-threatening complications such as renal failure, pulmonary embolism and septicemia. In addition, many SCI patients experience chronic conditions such as bladder dysfunction, pressure ulcers and pain, all of which severely impact quality of life. It can cost up to $5M to treat an SCI patient during their lifetime. SCI exerts a significant economic, emotional and physical toll on patients and their caregivers.
There are no treatments that can reverse the paralysis resulting from a SCI. Current options are geared towards minimizing associated complications. Furthermore, most treatments in development can enhance the survival of undamaged neurons, however, they do not result in the production of new functional neurons that can replace those lost as a result of SCI.
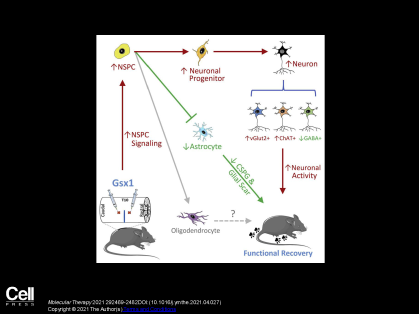
We have developed a novel, first-in-class GSX1 gene therapy that promotes the regeneration of spinal cord tissue, resulting in dramatic functional recovery in an animal model of spinal cord injury (SCI). This is the first treatment for SCI that enhances regeneration of spinal cord tissue, attenuates scarring and reprograms the spinal cord circuitry; all of which are essential for functional recovery. This therapy has the potential to help thousands of SCI patients regain motor control.
Market Applications:
- Treatment for spinal cord injury
- Promotes residential neural stem cell activation and cell proliferation
- Increases the generation of specific neurons
- Reduces glial scar formation
- Promotes functional recovery