Diagnostic Screening Assay for Tick-borne Infections in Donated Blood Samples
Inventor (s): Nikhat Parveen
Date Awarded: December 2019
Summary:
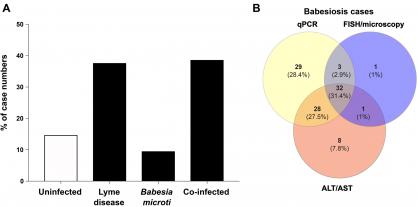
Confirmatory diagnostic assays for active tick-borne diseases are not yet fully established. We used our patented molecular beacon probe-based multiplex quantitative PCR (qPCR) assay to screen patient samples from New Jersey for three tick-borne infections, Anaplasma phagocytophilum, Babesia species and Borrelia burgdorferi. In addition to Lyme disease, we found a high prevalence of babesiosis, with co-infection case numbers similar to those reported in New York and Connecticut. Results of our assay were confirmed using alternative serological/FISH assays and liver-based enzymatic assays (aspartate aminotransferase, AST, and alanine aminotransferase, ALT). Studies have shown that babesiosis is the most common cause of mortality associated with blood transfusion in the United States. Because currently blood bank samples are not widely tested for Anaplasma and Babesia, asymptomatic infected patients keep donating blood, increasing the chance of transfusion-transmitted diseases. It is obligatory to test blood supplies for hepatitis B virus (HBV). Therefore, we will further expand our diagnostic assay to include HBV testing to ensure the safety of donated blood going forward.
Market applications:
- Stand-alone assay for clinical diagnosis of infection in individual patients
- For testing of veterinary samples (primarily dogs)
- Screening of blood bank samples